Ammm:Mm aplus
Introduction
One is recommended to read through and understand the background knowledge of polarizable force field AMOEBA, in order to have a better learning experience of the next generation AMOEBA+ force field. As indicated by the name, AMOEBA+ is based on the AMOEBA framework with incoporation of essential physical effects that are missing in AMOEBA model. With the short-range charge penetration and charge transfer effects being included, AMOEBA+ potential is able to follow the energy components by the Symmetry Adapted Perturbation Theory (SAPT).
Below brief description of the SAPT theory is introduced, followed by the specific interactions/physical effects that are incoporated in AMOEBA+ potential. In the last part, brief summary of the current status of the parametrization of the AMOEBA+ force field is given.
SAPT Method
Intermolecular interactions are small (comparing to the intramolecular interactions). For example, helium dimers at equalibrium only has interaction energy of -0.02 kcal/mol. However, the intermolecular interactions are important for describing a lot of phenomina in chemistry, physics and biology.
Starting from a dimer AB composed of two monomers, A and B, the interaction energy is computed as:
This is often called supermolecular approach to calculate intermolecuar energies. The total energy of each fragment is usually at least 4 orders of magnitude larger than the interaction energy. So this calculation is largely relies on error cancellation. In addition, this approach only gives one number of that interaction, which limits its interpretation ability. From a perturbative approach, SAPT is able to calculate the interaction energy accurately, meanwhile provides energy terms with physical interpretation.
The Hamiltonian of SAPT is constructed as follows:
where F is the Fock operator, W is the intranonomer correlation operator and V is the intermonomer interaction operator. In this approach, the intramonomer correlation is included via the second perturbation expansion involving the Møller-Plesset (MP) fluctuation potential. Interaction actions are then expressed as:
where i(j) is the orders of V(W). By grouping together different orders of the corrections above, SAPT decompose the interaction energy into four energy terms, namely, electrostatics, induction, exchange and dispersion:
The simplest SAPT approximation that gives reasonable total interaction energy is SAPT0, in which the intramonomer correlation is completely neglected. SAPT0 is sometimes useful for understanding the nature of interactions between relatively big fragments (hundreds of atom). The highest order of SAPT is obtained when i=3 and j=2, which corresponds to SAPT2+3. Alternatively, there are truncated levels of SAPT, including SAPT0, SAPT2, SAPT2+ and SAPT2+(3). The detailed hierachy grouping of SAPT theory can be seen below:
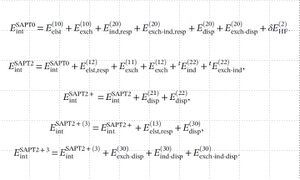
Finally, it is worth mentioning that there are other variations of SAPT approaches. In addition, there exist other energy decomposition analysis (EDA) methods. We will revist the SAPT induction contribution in the following section, from which we know that the polarization and charge transfer (CT) are folded together in SAPT. Many other EDA approaches try to separate CT from induction but the results are usually very different from each other.
New Potential Energy Terms
The AMOEBA force field utilizes the MM3-style valence terms. The intermolecular terms include permanent electrostatics, polarization and van der Waals interactions.
Although the valence interactions are important, in AMOEBA+ model we try to improve the intermolecular interactions
Charge Penetration
Polarization and Charge Transfer
Different EDA methods separate these two contributions very differently. For example, ...
Here we first tried to examine the many-body energy of AMOEBA model and compare with high-level quantum calculations.
The interaction energy of a system can be expressed as a sum of N-body interactions, where N is the number of monomers in the system. The magnitude of the interactions decrease as the order increases. The first many-body contribution is from 3-body. Practically, the contribution of 5-body and beyond is quite small comparing to lower orders. In our model, we focused on the 3-body and 4-body interaction energies. In a trimer cluster composed of three monomers A, B and C, the 3-body interaction energy can be calculated as the following, where the overline represent the existence of that monomer in the total energy calculation as dumming atoms. In this way, the BSSE is accounted natually. Similarly, one can get the 3-body and 4-body energy in a tetramer composed of monomers A, B, C and D. A detailed description can be found in the supporting information of this JCTC paper.[1]
New damping function
where
Finally, we provide a natural way to separate induction into polarization and charge transfer. The CT is then modeled using an empirical pairwise potential.
Geometry Dependent Charge Flux
Charge flux is necessary to capture the correct dipole moment surface of a molecule, which is critical to many properties, such as the geometry changes of molecules in different phases and spectroscopy properties. AMOEBA+ follows the formula proposed by Dinur and Hagler [ref]. The deviation of bond and angle from their equilibrium values will lead to charge "flux" from one atom to another.
Other Improvements
Polarizability
Van der Waals
Valence Parameters
Parametrization
References
- Embedded PDF only shows on browsers including FireFox, Edge and Chrome (sometimes not). IF you do not see it, please download it to read.









